How Many F Elements Are There?
The F elements, also known as the “inner transition metals”, are a group of 15 lanthanide elements and 5 actinide elements on the periodic table. They are located in the periodic table between the s-block and d-block elements. They are classified as inner transition metals because they fill the f orbital subshell, allowing the f block electrons contained with each member to act as valence electrons during chemical reactions.
Definition of F Elements
F elements are those elements in the periodic table whose last valence electron enters the f orbital of an atom. They belong to the inner transition metals and comprise all the lanthanides and actinides in the periodic table. F elements are special because they have an incompletely filled f orbital, which gives them unique atomic and chemical properties compared to other elements.
Specifically, the f orbital is an inner shell orbital that can hold up to 14 electrons. It is usually incompletely filled, unlike the s, p, d, and f orbitals of other elements. This incompletely filled f orbital is what defines f block elements and gives them properties like complex formation, paramagnetism, and color changes. The f electrons are also shielded from the atom’s surroundings, giving f block elements unique chemistry.
Discovery of F Elements
The first F element, fluorine, was discovered in the late 18th century by Carl Wilhelm Scheele, who called it “fluoric acid air.” Humphry Davy eventually proved it was an element in 1810 and named it fluorine, derived from the Latin word “fluere” meaning “to flow” due to its low melting point. Fluorine proved very difficult to isolate due to its extreme reactivity. Many early researchers died or were injured trying to work with fluorine.
Fermium, element 100, was synthesized in 1952 by a team of scientists led by Albert Ghiorso at the University of California, Berkeley. It was named after physicist Enrico Fermi. The discovery helped confirm Einstein’s theory of nuclear fission. Most other F elements have been artificially produced and were named after scientists.
Overall, the discovery of the F elements expanded our understanding of the periodic table and chemical elements. Their unique properties have led to many practical applications, despite the dangers of working with these highly reactive elements.
Total Number of F Elements
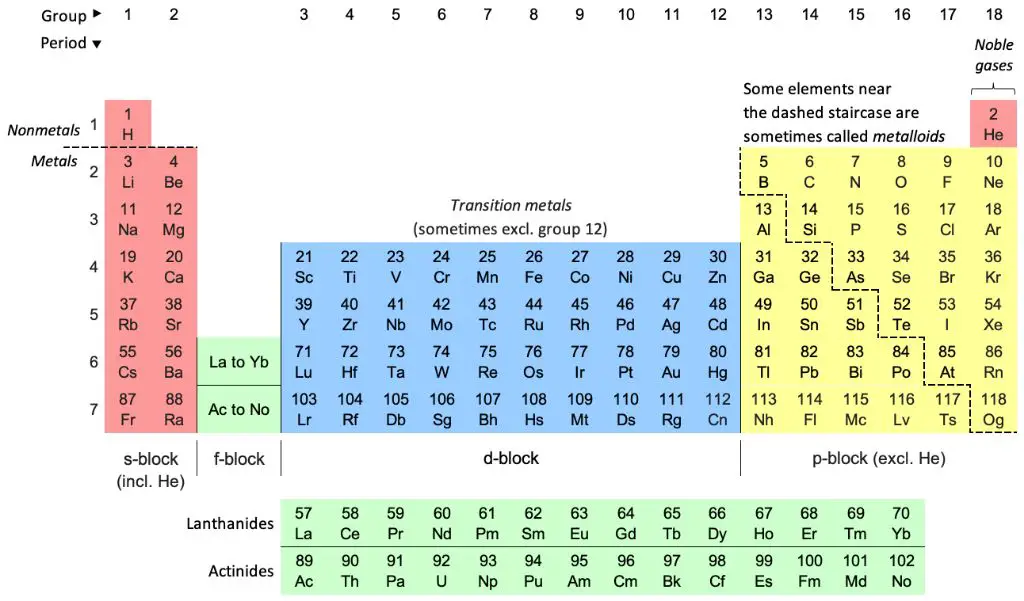
There are a total of 18 F elements discovered so far. They are the following:
- Fermium (Fm)
- Flerovium (Fl)
- Ununtrium (Uut)
- Ununquadium (Uuq)
- Ununpentium (Uup)
- Ununhexium (Uuh)
- Ununseptium (Uus)
- Ununoctium (Uuo)
- Ununennium (Uue)
- Unbinilium (Ubn)
- Unbiunium (Ubu)
- Unbibium (Ubb)
- Unbitrium (Ubt)
- Unbiquadium (Ubq)
- Unbipentium (Ubp)
- Unbihexium (Ubh)
- Unbiseptium (Ubs)
- Unbioctium (Ubo)
The F elements are radioactive metals that have atomic numbers from 100 onwards. They are all synthetic elements produced in laboratories. Due to their radioactive nature, none of the F elements have any stable isotopes.
Groups Within F Elements
The F elements are divided into two groups based on their electronic configuration – the lanthanides and the actinides.
The lanthanides consist of the 15 elements with atomic numbers 57 through 71, from lanthanum (La) to lutetium (Lu). They fill the 4f subshell progressively from cerium (Ce) to lutetium. The lanthanides are also known as the “rare earth” elements.
The actinides consist of the 14 elements with atomic numbers 89 to 102, from actinium (Ac) to lawrencium (Lr). They fill the 5f subshell progressively from thorium (Th) to lawrencium. The actinides are all radioactive.
The groups together form the inner transition metals. They are called “inner transition” metals because they provide transitions between the s- and d-blocks of the periodic table. The f-orbitals of their valence shells are inner orbitals, shielded from interaction with the environment by the outer s and p electrons. This shielding gives them unique properties compared to other elements.
Both the lanthanides and actinides are placed below the main body of the periodic table due to their filled or partially filled f-orbitals. The lanthanides and actinides demonstrate similar properties within their groups, but differ from each other in reactivity and other physical and chemical properties.
Source: https://study.com/learn/lesson/f-block-elements-on-the-periodic-table-properties-lesson.html
Natural Occurrence
The F elements are not naturally abundant on Earth. The most common naturally occurring F element is fluorine, which ranks 25th in elemental abundance in the Earth’s crust. Fluorine is found in minerals like fluorite, fluorspar and cryolite, but is also rather widely distributed in other minerals. It is the 13th most abundant element in seawater. Fluorine is produced in the interiors of certain stars through nuclear fusion reactions. Elements heavier than fluorine are considered rare.
The heavier F elements are not found naturally on Earth due to their instability and radioactive decay. Elements like neptunium and plutonium are produced artificially in nuclear reactors or particle accelerators. The most stable isotope of plutonium, plutonium-244, has a half-life of 80 million years. The radioactive decay of uranium and thorium produces small amounts of heavier actinides like protactinium, uranium, neptunium and plutonium. Trace quantities of some heavier elements may be found in uranium or thorium ores. Overall, the heavy F elements have extremely low natural abundance.
Sources:
https://en.wikipedia.org/wiki/Origin_and_occurrence_of_fluorine
https://www.rsc.org/periodic-table/element/9/fluorine
Properties
The f-block elements, also known as inner transition metals, are unique due to their electron configurations. They have partially filled f orbitals in their ground state or valence electron configuration 1. This gives them distinct chemical and physical properties compared to other elements.
In general, f-block elements tend to have high melting and boiling points due to strong metallic bonding between atoms. For example, tungsten, which is an f-block element, has the highest melting point of any pure metal at over 3400°C 2.
They also exhibit high densities, with osmium being the densest naturally occurring element. Many f-block elements are radioactive due to their nuclear instability 3. Additionally, they are mostly reactive metals, although the degree of reactivity varies across the f-block.
The lanthanides demonstrate paramagnetic properties in compounds due to unpaired electrons in the 4f orbitals. Meanwhile, the actinides are generally more reactive than the lanthanides. Overall, the distinctive electronic configurations of the f-block elements lead to their unique chemical and physical behaviors.
Uses
The f-block elements, also known as the lanthanides and actinides, have a variety of uses in industry, medicine, and technology. Some common uses include:
Magnets – Many lanthanide elements like neodymium and samarium are used in strong permanent magnets. These magnets are found in a range of products like headphones, motors, generators, and MRI machines (Nature).
Alloys – Lanthanides can be added to steel and other metals to improve strength, heat resistance, and other properties. For example, cerium is used as an alloying element for steel (Toppr).
Ceramics – Compounds of lanthanides and actinides give color to ceramics and enamels. Europium is used in red phosphors in TV sets (GeeksforGeeks).
Lasers – Many lanthanide elements are used in fiber optic lasers and other solid-state laser crystals. Erbium-doped fiber amplifiers are important for long-distance fiber optic communications (Nature).
Nuclear industry – Actinides like uranium and plutonium are used as nuclear reactor fuel. Thorium shows promise as a nuclear fuel for next-generation reactors (Toppr).
Biological Role
Fluorine, while not essential for human life, does play an important biological role. Fluoride, the ionic form of fluorine, has been shown to help strengthen teeth and bones when consumed in small amounts. According to Wikipedia, fluoride is considered a “semi-essential element” for humans since it contributes to dental health but is not necessary to sustain life.
The Royal Society of Chemistry states that “Fluoride is an essential ion for animals, strengthening teeth and bones. It is added to drinking water in some areas.” Fluoride helps prevent tooth decay by making tooth enamel more resistant to acid attacks from plaque bacteria. It also promotes remineralization, which aids in repairing early decay.
While fluorine itself does not play a biological role, its ionic form fluoride certainly does. Consumption of the proper amount of fluoride contributes to good dental health. However, too much fluoride can also have detrimental effects, so intake levels need to be monitored.
Conclusion
The F elements, also known as inner transition metals, consisting of lanthanide and actinide series are critical components of modern technology and have a wide range of applications. They exhibit unique chemical and physical properties due to their partially filled f orbitals. While only a few of the lanthanide elements naturally occur on earth, several actinide elements were first synthesized in laboratories. Highlights about the elements include:
- There are two series within the F block – 15 lanthanides and 14 actinides, with the lanthanoids lighter than actinoids.
- Lanthanides are vital components of magnets, lasers, phosphors, and help catalyze reactions.
- Actinides like uranium and plutonium are important energy sources and are used in nuclear reactors.
- Promethium is one of the two elements that do not occur naturally.
- Fluorine is the most electronegative and reactive of all elements.
- F elements display variable oxidation states and complex chemistry.
In summary, the unique properties of the f-block elements make them indispensable modern commodities, despite their limited natural occurrence. Their vital applications in technology, medicine, energy, and more underscore the significance of continuing research on these elements.



