How Do You Write Cu2O?
Cu2O, also known as cuprous oxide, is an inorganic chemical compound with the formula Cu2O. It is composed of copper and oxygen atoms, with copper in the +1 oxidation state. Cu2O has a red or brownish-red color and is usually found as a powder or crystalline solid.
Some key properties of Cu2O include:
- It has a cubic crystal structure.
- It is a p-type semiconductor.
- It has a band gap of 2.17 eV.
- It exhibits photoconductivity and photoluminescence.
- It is used as a positive electrode material in lithium ion batteries.
- It can be used as a catalyst and pigment.
Chemical Formula
The chemical formula for cuprous oxide is Cu2O. This means that each molecule of cuprous oxide contains 2 copper (Cu) atoms and 1 oxygen (O) atom.
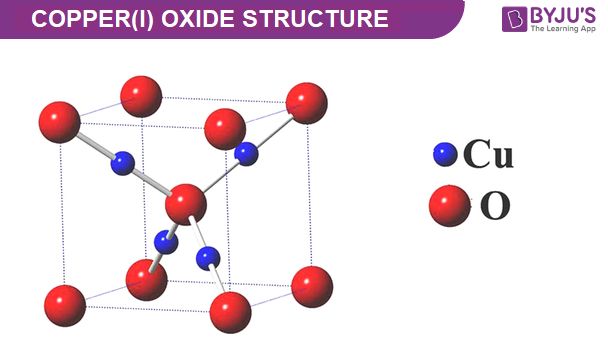
The subscript 2 next to the Cu indicates that there are 2 atoms of copper in the formula. The subscript 1 is omitted after the O since it is understood that there is 1 atom of oxygen if no subscript number is present.
So in summary, the chemical formula Cu2O tells us that cuprous oxide consists of 2 copper atoms bonded to 1 oxygen atom in each of its molecules. This precise 2:1 ratio of Cu:O atoms gives cuprous oxide its distinctive properties.
For more details see: https://studylib.net/doc/7517184/chemistry–chemical-bonding-activity
Molecular Geometry
The molecular geometry of Cu2O is linear. It consists of a central copper atom with two oxide ions bonded on opposite sides, forming a linear geometry along a 180° angle. The copper atom has a +1 oxidation state and each oxygen atom has a -2 oxidation state. The two Cu-O bonds are covalent bonds.
The formal electron pair geometry is linear since there are two bond pairs around the central copper atom. With no lone pairs, the molecular geometry matches the electron pair geometry and is linear. The copper atom has a filled 3d10 subshell and the oxygen atoms have filled 2p6 subshells. This results in a Cu+1 ion and two O-2 ions which bond together to satisfy the octet rule.
The linear geometry of Cu2O can be represented by the chemical formula O-Cu-O and is an example of a simple covalent network solid with a non-molecular structure. The linear geometry maximizes distance between the negatively charged oxide ions. Overall, Cu2O has a symmetrical linear molecular geometry consisting of a Cu+1 ion bonded to two O-2 ions.
Oxidation States
Cu2O, known as cuprous oxide, contains both copper (Cu) and oxygen (O) atoms. In Cu2O, the copper mainly exists in a +1 oxidation state, while the oxygen exists in a -2 oxidation state.
The +1 oxidation state for copper is known as the cuprous state. This is in contrast to the more common +2 oxidation state for copper, known as the cupric state. The cuprous +1 state is stabilized in Cu2O due to the closed shell d10 electronic configuration.
Meanwhile, oxygen nearly always takes a -2 oxidation state in its compounds. Each O2- oxide ion bonds to a Cu+ cation in the Cu2O structure.
Overall, the charges balance out in Cu2O, with the +1 oxidation state copper combining with the -2 oxidation state oxygen to produce a neutral compound.
Physical Properties
Some key physical properties of cuprous oxide (Cu2O) include:
Color: Cu2O has a red or reddish-brown color in its natural mineral form known as cuprite. The synthetic material also appears as a red-orange to red powder.
Density: The density of Cu2O is 6.0 g/cm3. This is lower than the density of 9.0 g/cm3 for CuO, the other common oxide of copper.
Melting Point: Cu2O has a melting point of 1,232°C (2,250°F). This high temperature makes it useful for certain high-temperature applications.
Additional physical properties include a cubic crystal structure, a bandgap of 2.172 eV, and semiconductor behavior. Cu2O can transition from a p-type semiconductor to an n-type semiconductor depending on the synthesis method and presence of defects in the crystal lattice [1].
Chemical Properties
Cu2O has moderate chemical reactivity and solubility. It dissolves slowly in acids to release cupric ions (Cu2+). For example, Cu2O will react with hydrochloric acid (HCl) to produce copper chloride (CuCl2) and water (H2O) (1):
Cu2O + 2HCl → 2CuCl2 + H2O
Cu2O also reacts with sulfuric acid (H2SO4) to form copper sulfate (CuSO4) and water (2):
Cu2O + H2SO4 → CuSO4 + H2O
It has low solubility in water but dissolves in ammonia to form the complex ion tetraamminecopper(II) ([Cu(NH3)4]2+) which has a dark blue color (3). Cu2O exhibits amphoteric properties, meaning it can react with both acids and bases. For example, it dissolves in sodium hydroxide (NaOH) to produce copper(II) hydroxide (Cu(OH)2):
Cu2O + 2NaOH → Cu(OH)2 + Na2O
Overall, Cu2O displays moderate reactivity that is dependent on factors like particle size, morphology, exposed crystallographic planes, and more (4).
[1] https://www.researchgate.net/publication/259629104_Chemical_Deposition_of_Cu2O_Nanocrystals_with_Precise_Morphology_Control
[2] https://chemistry-europe.onlinelibrary.wiley.com/doi/pdfdirect/10.1002/cphc.201300735
[3] https://chemistry-europe.onlinelibrary.wiley.com/doi/pdfdirect/10.1002/cphc.201300735
[4] https://www.researchgate.net/publication/259629104_Chemical_Deposition_of_Cu2O_Nanocrystals_with_Precise_Morphology_Control
Synthesis
Cu2O can be synthesized through various methods including oxidation, electrodeposition, and solution-based techniques. A common approach is the oxidation of copper metal or salts in alkaline solutions containing glycerol, ethylene glycol, or glucose which act as reducing agents (https://www.sciencedirect.com/science/article/pii/S0925838820309865). Electrodeposition is also used, where copper sheets or foils are immersed in electrolyte baths containing cupric salts and ligands like ethylenediamine or polyethylene glycol to control morphology and crystallinity (https://pubs.acs.org/doi/10.1021/ja209662v). Solution-based methods like hydrothermal, solvothermal, and polyol synthesis allow shape and size control by using surfactants and capping agents in the presence of heat and pressure.
Applications
Cu2O has a wide variety of applications in the fields of energy conversion, the environment, and electronics due to its promising properties. Some of the key applications of Cu2O include:
Gas sensing – Cu2O is often used as a sensing material for detecting hazardous gases such as carbon monoxide (CO), ammonia (NH3), and nitrogen oxides (NOx) due to its high sensitivity and stability. The conductivity of Cu2O changes upon interaction with different gases, allowing it to be used in gas sensor devices [1].
Photocatalysis – The photochemical properties of Cu2O make it useful as a photocatalyst for applications like water splitting to produce hydrogen fuel and degradation of organic pollutants in wastewater treatment. Under light irradiation, Cu2O can catalyze redox reactions on its surface [1].
Solar energy conversion – Cu2O has been used in photovoltaic cells and solar-to-fuel conversion systems as an inexpensive and earth-abundant light absorber. Its bandgap allows it to absorb visible light for conversion into electrical or chemical energy [2].
Electronics – The p-type semiconductor properties of Cu2O make it useful in electronic applications like diodes, transistors, sensors, and memristors. It has also been incorporated into resistive random-access memory devices [1].
Health and Safety
Cu2O can potentially pose some health and safety hazards that should be considered when handling it. According to the material safety data sheet from ESPI Metals, dust from Cu2O should be avoided as it can be irritating if inhaled. The dust can also irritate the eyes and skin on contact. Ingestion of Cu2O is also considered harmful. Proper protective equipment like gloves, goggles, and a mask should be worn when handling the chemical. Workspaces should also be equipped with adequate ventilation systems if dust is generated during handling.
The University of Illinois’ safety data sheet additionally warns that Cu2O can oxidize to copper(II) oxide at high temperatures above 100°C. The potential formation of this irritating and toxic compound should be considered when handling Cu2O at elevated temperatures. Overall, caution should be exercised when working with Cu2O to minimize exposure and control dust.
Conclusion
In summary, Cu2O, also known as cuprous oxide, is an inorganic compound with the chemical formula Cu2O. It is a red or reddish-brown solid semiconductor material composed of copper and oxygen atoms. Cu2O has a simple cubic crystal structure and contains copper in the +1 oxidation state. It exhibits both metallic and non-metallic properties and has relatively high optical bandgap energy. Some key properties and applications of Cu2O include:
– Acts as a p-type semiconductor due to copper vacancies
– Photoconductive and photoluminescent due to its bandgap
– Used in solar energy conversion as an absorber material in solar cells
– Used as a photocatalyst for water splitting and degradation of organic pollutants under visible light
– Potential use in gas sensors and lithium-ion batteries
– Biocompatible and non-toxic, allowing use in biological applications
– Can be synthesized through oxidation of copper metal, thermal decomposition, electrodeposition, and other methods
In summary, Cu2O is a versatile semiconductor material with many current and potential uses in renewable energy, catalysis, electronics, and biomedicine due to its unique optical, electrical, and physical characteristics.