Clay Composition: Unveiling Different Types And Formulations
Clay is a natural material composed primarily of fine-grained minerals, which show plasticity and harden when fired. The most common clay minerals are kaolinite, montmorillonite-smectite, illite, and chlorite (Clay). Clays develop plasticity when mixed with water, due to the layer lattice structure of the clay minerals allowing water molecules to enter between the layers. This creates a flexible, moldable material that holds its shape when dried.
Clays have been important materials since the dawn of civilization. Prehistoric humans discovered the useful properties of clays and used them to make pottery and artworks. The earliest known clay ceramics date back over 14,000 years in East Asia (Clay and Pottery Brief History). Ancient civilizations utilized clays to construct buildings, make cookware and storage vessels, fashion figurines, and more.
Today, clays remain indispensable across many industries. Their unique chemistry lends well to applications ranging from ceramics, construction materials, paper, rheology modifiers, absorbents, and environmental remediation. Clays will continue serving critical roles thanks to their abundance, affordability, and versatility.
Clay Mineral Groups
The four main groups of clay minerals are kaolinite, smectite, illite, and chlorite. Each group has distinct composition and properties that lend themselves to certain uses.
Kaolinite has a 1:1 layer structure, meaning it consists of alternating silica tetrahedron and alumina octahedron layers. It is non-swelling and stable at high temperatures. Kaolinite is the main component of kaolin or “china clay”, which is used for making porcelain and fine china. Its non-swelling properties make it useful in paper coating and filler applications [1].
Smectites, including montmorillonite, have a 2:1 expanding lattice structure with charges that attract polar molecules like water. This allows smectite clays to absorb large amounts of water and swell considerably. Their absorbent properties make smectites useful as animal feed supplements, pesticide carriers, and oil and grease absorbents [2].
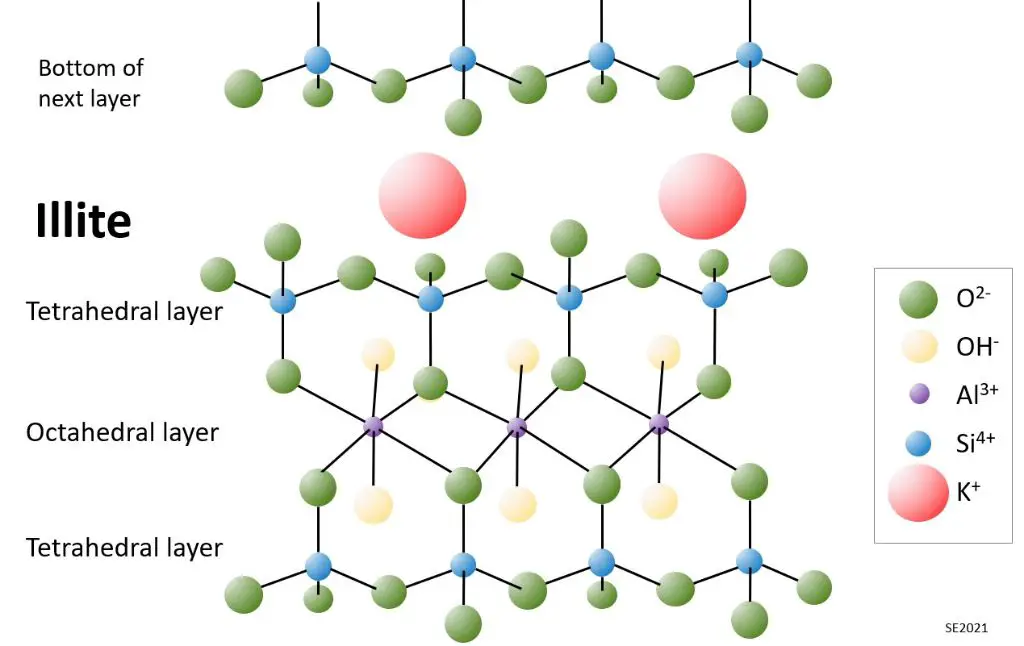
Illite has a non-expanding 2:1 structure similar to smectite but with different layer charges that limit swelling. Illite rich clay bodies are used for stoneware and porcelain ceramics. Illite’s weathering properties also make it a major component of shale mudstone and slate [1].
Chlorite clays have a 2:1:1 lattice comprising two tetrahedral sheets surrounding an octahedral sheet. Chlorite’s negative charges attract and fix positively charged compounds like heavy metals. This gives chlorite absorbent and catalytic properties used in waste treatment and petroleum cracking [2].
Plasticity and Stickiness
Plasticity refers to the ability of clay to be shaped or molded without cracking or crumbling. According to DigitalFire, plasticity is dependent on the amount of water mixed into the clay. With increased water content, clay becomes more plastic and sticky as the water molecules attach to the clay particles and act as a lubricant, allowing them to slide over one another.
The stickiness of clay increases as more water is added. Very sticky or “gumbo” clays contain high amounts of water and can cling to surfaces. However, research shows that plasticity also depends on the clay mineral composition. Clays high in montmorillonite tend to be more plastic and sticky due to the structure and electrical charges of its platelets. Less plastic kaolin clays have a lower water content at the plastic limit.
Firing and Vitrification
Firing clay is the process of exposing it to high temperatures in a kiln, which causes chemical and physical changes that alter the clay permanently. Firing has three main stages:
- Drying – Clay must be completely dry before firing or else it can explode in the kiln. Drying occurs around 212°F.
- Bisque Firing – The initial firing turns the clay into a permanent ceramic material by removing any remaining water and organic matter. Bisque firing occurs between 1,832-2,102°F.
- Glaze Firing – The final firing melts glass-like glazes onto the bisque ware to create a glossy coating. Glaze firing occurs between 2,190-2,462°F [1].
During firing, clays undergo vitrification, which is the fusion of clay particles under intense heat. As temperature rises, the clay transitions from porous and weak to dense, durable, and non-absorbent. Complete vitrification occurs between 2,100-2,300°F when the clay has fully melted. The degree of vitrification depends on the clay composition, kiln temperatures, and length of firing [2].
Pottery Clay Bodies
Pottery clay bodies can be categorized into three main types based on their mineral composition and firing temperatures: earthenware, stoneware and porcelain.
Earthenware clay contains a high percentage of iron and other minerals. It has a coarse texture and requires firing at lower temperatures, typically between 1,800-2,100°F. Earthenware clays are very plastic and smooth but absorbent in nature, making them ideal for handbuilding and sculpture but less suitable for functional ware. Common earthenware clay types include red and brown clays (such as terracotta), some fire clays, and casting slip clays like MO-CLAY.
Stoneware clay has lower iron content and requires firing at high temperatures between 2,200-2,400°F to fully vitrify and become watertight. It contains minerals that flux at these temperatures, resulting in a strong, nonporous product that is suitable for functional wares. Typical stoneware clay is smooth, dense and plastic. Common types include black, buff, red and white stoneware clays. (1)
Porcelain clay bodies have a very high percentage of kaolin clay. They are fired at ultra-high temperatures from 2,200-2,450°F and become completely vitrified, resulting in a translucent white clay that is nonporous and very strong. However porcelains can be difficult to work with due to lack of plasticity. Small amounts of feldspar, silica and ball clay are often added to improve workability. Porcelain clays are ideal for functional tableware, electrical insulators and technical ceramics.
Clays can be modified into clay bodies by adding non-plastic materials as fillers and fluxes. Common additives include feldspar, flint, silica, grog and talc. These serve to open up the clay body, improve drying behavior and firing qualities, and alter technical properties like shrinkage, plasticity, color and texture. (2)
Sculptural Clays
Sculptural clays need to have properties that make them easy to shape and mold into artistic creations. Some key properties include:
Plasticity – The ability to be molded and hold shape. More plastic clays are easier to sculpt intricate details with.
Stickiness – Stickier clays hold their shape better without slumping or sagging.
Strength – Clays need enough strength when dry to support the weight of the sculpture without cracking or crumbling.
Low Shrinkage – Minimal shrinkage when drying prevents cracking and distortion.
The most common types of sculptural clays include:
Oil-based clays – Offer excellent plasticity and stickiness. Top brands include Chavant NSP, Van Aken Plastalina, Monster Clay, and Roma Plastilina.
Polymer clays – Don’t require firing and dry at low temperatures. Popular brands are Sculpey and Fimo.
Water-based clays – Easy to work with and good for beginners. Leading options are Newclay and Pottery Clay.
Air-dry clays – Convenient choice as they dry at room temperature. Good brands are Crayola Air Dry Clay and Das Clay.
Clay Slip
Clay slip, also known as liquid clay or simply slip, is a liquid mixture of clay and water that has a creamy consistency. According to thesprucecrafts.com, “The term slip in making pottery means a suspension of clay particles in water.” [1] Slip is an essential material in pottery and ceramic work.
The main ingredients in clay slip are clay, water, and sometimes deflocculants or additives. The clay is suspended in water at a ratio that achieves the desired viscosity for the slip’s intended use. Deflocculants are added to prevent clumping and help keep the clay particles suspended evenly. Other additives like grog, vermiculite, or metal oxides may be included to modify the slip’s properties.
There are several common uses for clay slip in pottery and ceramics:
- Applying colored slips to bisqueware for decoration and then firing again to set the color.
- Using slip trailing for adding raised decorative details.
- Acting as “glue” for joining pieces of unfired clay.
- Coating the interiors of pieces to make them less porous and give a smooth surface.
- Pouring into plaster molds for slipcasting.
Clay slip is an indispensable material for potters and ceramic artists. Its fluid properties allow it to be used in various forming and decorating techniques to create beautiful finished wares.
Clay Materials Testing
Testing clay materials is an important part of selecting the right clay for a specific project or purpose. Some key properties to measure include plasticity, shrinkage, absorption, and strength. Certain tests can be performed simply at home, while others require more sophisticated laboratory equipment.
Plasticity refers to the ability of clay to be shaped or molded without cracking or crumbling. A simple test is the “snake” test – roll out a snake shape from a lump of clay and see how long it can be made before breaking apart. Highly plastic clays can be stretched into long thin snakes.
Shrinkage refers to the reduction in size as clay dries and fires. Test shrinkage with a shrinkage ruler – form a slab, cut a sample, and compare the wet and dry size. Typical ceramic clay shrinks 8-12% from wet to bisque fired state. Shrinkage should be minimized in clay used for precision items.
Absorption is the amount of water clay will soak up when immersed. Absorption relates to porosity and vitrification. Test absorption by weighing a dry test piece, soaking it in water, patting off surface moisture, and weighing again wet. Lower absorption indicates higher fired strength.
Strength indicates fired durability. Test bars can be formed, dried, and fired to measure modulus of rupture – the force required to break the bar. Durability is critical for structural ceramics and porcelain. Sophisticated tests can determine compressive strength, hardness, and wear resistance.
Understanding a clay’s characteristics through testing allows proper selection as an modeling clay, pottery clay, or structural clay. Consistency between batches can also be verified. For more details on clay testing procedures, visit the Digitalfire and Ceramic Arts Network articles on clay testing.
Clay Safety
When working with clay, it’s important to follow proper safety practices to avoid exposure to potentially toxic materials.
According to the Safety Rules for Ceramics from South Texas College, firing clay and glazes produces harmful gases like carbon monoxide, formaldehyde, and sulfur dioxide. Chronic inhalation of silica dust from clay mixing can also cause lung disease known as silicosis or “potter’s rot.”
To prevent exposure, ceramics studios must have proper ventilation and dust collection systems. When mixing dry clay, it’s recommended to wear an N95 respirator mask. Work surfaces should be kept clean and wet mopped to minimize airborne dust. Materials containing silica or other toxins should be handled carefully and gloves worn during use. Any contaminated clothing should be washed separately.
Following basic studio safety rules and using proper protective equipment can help minimize the health risks associated with clay materials. Proper ventilation, cleaning, and handling practices are key to promoting a safer ceramics workspace.
Innovations in Clay
The clay industry has seen exciting innovations in recent years as companies seek to develop more efficient, sustainable, and high-performance clay products and manufacturing processes. Some notable innovations include:
New clay 3D printing technologies are enabling more intricate designs and complex geometries for pottery and other clay products that were previously not possible (1). Companies like 3D Potter are using clay 3D printing to create customized pottery.
Researchers have developed new clay aerogel materials that are ultra lightweight yet very strong, which could enable lighterweight ceramics for applications like insulation and packaging (2). Clay aerogels are made by extracting the water from clay gels.
Some companies are exploring the use of clay as a renewable and sustainable packaging material to replace plastics. Researchers at UCLA have developed a process using clay and chitin from shellfish to create flexible food packaging (3).
Clay mineral companoes are developing new engineered clays to enhance properties like stability and absorption capacity for applications ranging from ceramics to cat litter to pharmaceuticals.
Looking ahead, some see potential to use clay for high-tech applications like batteries, semiconductors, and pollution control. Continued innovation and R&D will open up new uses for this abundant and versatile material.



