What Makes Clay Dry Fast?
Clay is a unique material composed of fine mineral particles that can be molded when wet, hardened when fired at high temperatures, and re-softened later with the addition of water. Knowing the factors that make clay dry fast is important for potters and ceramic artists who want to efficiently create pieces without cracking or deforming.
There are several variables that impact the drying time of clay. The chemical composition and particle size influences how quickly moisture evaporates from the clay body. Environmental factors like temperature, air circulation, and humidity play a key role. The thickness and shape of the clay piece also matters. Finally, different drying techniques and additives can shorten drying time. Understanding how these factors work together enables faster and safer clay drying.
Chemical Composition
The chemical composition of clay has a significant impact on how quickly it dries. Clays contain a variety of minerals like silica, alumina, iron, lime, magnesia, potash, soda and titania. The most common clays contain a high percentage of silica and alumina. The more silica a clay contains, the slower it will dry. This is because the silica molecules hold onto water molecules more tightly through hydrogen bonding. Clays high in alumina will dry faster as the water molecules are not as strongly attracted. The iron, magnesium and calcium content also play a role. Higher iron leads to slower drying, while higher magnesium and calcium content speeds up drying. Understanding the mineral analysis of a clay body will give insight into its drying behavior.
Clay Particle Size
The size of the clay particles has a direct impact on how quickly clay dries. Clays are composed of tiny plate-shaped particles. The smaller the particles are, the more surface area is exposed to the air. With more surface area exposed, moisture can evaporate from the clay more quickly.
For example, a clay body made up of very fine particles, like porcelain, will dry faster than a clay with larger particle sizes, like stoneware. The porous nature of smaller clay particles allows moisture to escape rapidly through absorption and evaporation.
On the other hand, larger particles have less exposed surface area in relation to their volume. So it takes longer for the moisture to fully evaporate. Additionally, the larger particles leave wider spaces between them, resulting in a more open clay structure. This openness counterintuitively slows the drying process as moisture becomes trapped within the clay body.
Clay Thickness
Clay comes in varying thicknesses, from very thin to quite thick. Thinner clays generally dry faster than thicker clays. This is because with a thinner clay body, there is less material that needs to lose moisture in order to dry. The moisture in a thin clay slab has less distance to travel to reach the surface and evaporate. In a thicker clay body, there is more clay for the moisture to travel through before reaching the surface. The increased distance slows down the drying process.
For example, a clay slab rolled out to 1/4 inch thickness will dry much faster than the same clay rolled out to 1 inch thickness. The moisture only needs to travel 1/8 inch to the surface in the thinner slab, versus 1/2 inch in the thicker slab. With less distance to travel, the moisture evaporates faster, resulting in a faster overall drying time.
When working with clay, keep thickness in mind. If you want your clay to dry quickly, aim to keep the thickness lower. For pieces that don’t require much structural integrity, thin slabs down to 1/4 inch or less. For sturdier pieces, a slab thickness around 1/2 inch tends to offer a good balance between drying time and strength.
Drying Temperature
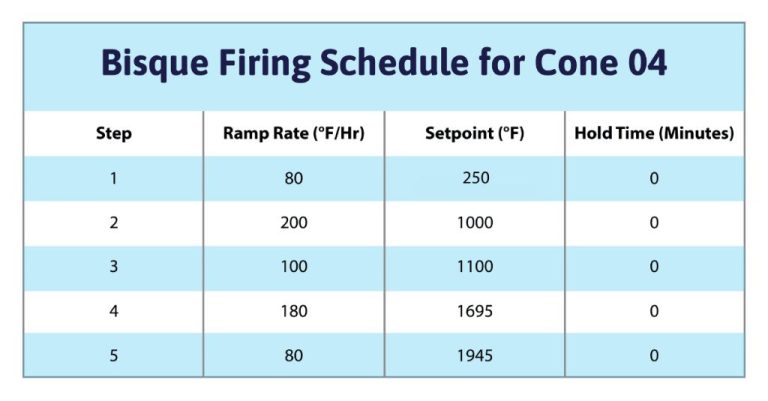
Higher temperatures accelerate the drying process of clay. This is because heat provides the energy needed for water molecules in the clay to transition from a liquid to a gaseous state through evaporation. At room temperature, which is around 70°F or 20°C, water evaporates from clay slowly. However, as the temperature rises, the molecules gain more kinetic energy and evaporate faster. Ideally, clay should be dried between 110-200°F to speed up drying time. Warmer studio spaces or use of kilns, ovens, or heat lamps creates an environment for faster evaporation. The higher the temperature (up to 200°F), the quicker the clay will dry. However, extremely high temperatures can lead to cracking or damage, so a balance is needed.
Air Circulation
One of the most important factors that affects how quickly clay dries is air circulation. Clay dries through the process of evaporation, where water molecules on the surface evaporate into the surrounding air. This evaporation happens much faster when there is moving air across the surface of the clay.
Fans or open windows that create airflow across clay will significantly speed up the drying process. The moving air molecules collide with and pick up water molecules on the surface of the clay. The more circulation the faster the moisture gets carried away. Stagnant air causes evaporation to happen at a much slower pace.
Increasing air circulation is an easy and effective way to accelerate the drying of clay. Positioning a fan to blow air across the clay or ensuring ventilation through open windows, doors, or vents will allow moisture to evaporate quickly so the clay fully dries faster. Proper air circulation slashes drying times.
Humidity
Humidity is a key factor in how quickly clay dries. Clay absorbs moisture from the surrounding air. In conditions with lower humidity, there is less moisture in the air for the clay to absorb. This allows water within the clay to evaporate more rapidly. Higher humidity slows down the evaporation of water from the clay. The more moisture in the air, the harder it is for the water in the clay to evaporate. This is because there is less of a moisture gradient between the wet clay and the surrounding air in high humidity conditions. The moisture wants to reach an equilibrium between the clay and the air. Lower humidity creates a greater moisture difference, making it easier for the water in the clay to evaporate into the drier air.
Clay Additives
Certain additives mixed into clay can significantly impact its drying time. One of the most common additives is grog, which is made from previously fired clay that has been ground into a powder. Grog particles help open up the clay body, creating pathways for moisture to escape. This allows wet clay containing grog to dry faster compared to plain clay. Adding as little as 10% grog by weight can cut the total drying time by more than half. Other common additives that speed drying include milled clays, silica, feldspar, and some plant materials. These all help absorb moisture, aiding water evaporation from the clay. The more porous the clay body becomes due to additives, the less time it takes to dry out.
Drying Method
There are several techniques that can be used to dry clay faster. Each method has advantages and disadvantages that impact drying speed:
Air Drying: Letting clay air dry at room temperature is the slowest method. Without additional heat, air circulation, or moisture absorption, air drying alone can take days or weeks for thicker pieces. Air drying is slow but safe for items prone to cracking and requires no special equipment.
Oven Drying: Using an oven or dedicated clay dryer to apply gentle heat speeds drying dramatically. Temperatures between 120-200°F are ideal, as higher heat can cause cracking. Oven drying thick items in hours instead of days, but energy costs are higher and cracking risks increase.
Heating Lamps: Positioning heat lamps or other directed heat sources towards drying clay helps accelerate moisture evaporation. Care is needed to prevent overheating. Heating lamps provide more rapid drying than air alone.
Dehumidifiers: Reducing ambient humidity around drying clay will significantly increase drying speed. Dehumidifiers work best in enclosed spaces like drying cabinets. Lower humidity allows moisture to evaporate from clay more quickly.
Blotting and Wicking: Absorbing surface moisture from clay physically pulls water out. Blotting with towels or wicking moisture into newsprint speeds drying by removing liquid water. Most effective when used in combination with other methods.
Conclusion
In summary, there are several key factors that determine how quickly clay will dry:
– Chemical composition: Clays with higher sand content tend to dry faster than clays rich in expandable minerals like montmorillonite.
– Particle size: Clays with smaller particles offer more surface area, allowing moisture to evaporate more quickly.
– Thickness: Thinner pieces of clay will dry faster than thick, dense pieces.
– Temperature: Warmer temperatures speed up the evaporation of moisture from clay.
– Air circulation: Good airflow across the clay’s surface improves moisture evaporation.
– Humidity: Low relative humidity allows moisture to evaporate from clay more readily.
– Additives: Adding dry materials like grog or sand can help improve air circulation in the clay body.
– Drying method: Exposing the clay to direct sunlight or using mechanical drying methods like fans or dehumidifiers accelerates drying.
By understanding these key factors, potters can adjust their clay preparation and drying conditions to achieve faster drying times.